
Biological processes
J.S. Gulliver, B.N. Wilson, O. Mohseni, A.J. Erickson, and R.M. Hozalski
Degradation of organic matter
Process: Several biological processes are involved in pollutant retention. The first is microbial respiration, in which organic matter in water is oxidized to CO2 which is shown in general form by equation 2.5, where CH2O represents organic matter.

Assessment considerations: In practice, the amount of readily degradable organic matter is quantified as biochemical oxygen demand (BOD). Oxidation of BOD produces CO2 and water. The BOD decay constant, “k” (equation 2.6), is dependent upon temperature. Although the magnitude of k is dependent upon bacterial population, the temperature effect on decomposition is generally described as shown in equation 2.7. Thus, the decay constant can be adjusted for different temperatures, as long as the bacterial population is similar.

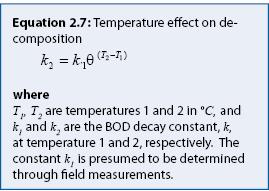
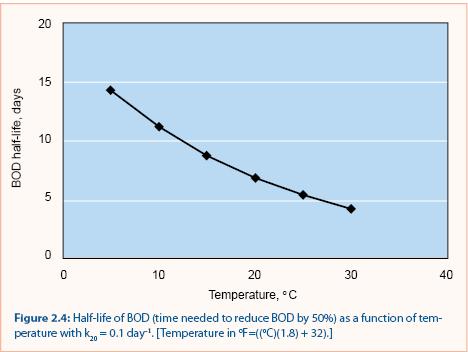
A common value for the empirical constant (θ) is 1.05 (Metcalf and Eddy 1991). Calculations using this value in equation 2.7 show that it takes three times longer to achieve a 50% BOD reduction at 5 °C (41 °F) than at 25 °C (77 °F) (figure 2.4). This implies that BOD decay would be least effective during the snowmelt period, when temperatures are just above freezing.
Denitrification
Process: Denitrification is a bacterial reaction that occurs under anaerobic (no dissolved oxygen) conditions, which are typical in sediments. Denitrification converts nitrate (NO3-) in stormwater to nitrogen gas (N2), as described in equation 2.8. Organic matter (again represented as CH2O) “fuels” the denitrification process; denitrification cannot occur without a source of organic matter.

Assessment considerations: Nitrate (NO3-) is generally less than one-third of the total nitrogen in urban stormwater (table 2.1). Denitrification can therefore remove only about one-third of stormwater nitrogen, unless additional nitrate is produced by nitrification (oxidation of ammonia). The end products are harmless gases.
These conditions occur in wetlands, where rooted plants supply the carbon, and in pond sediments, where carbon is supplied by dead algae. If the assessment program reveals that nitrate removal efficiencies are less than desired, assessment of the organic carbon supply may be warranted. As with other biological processes, denitrification is also controlled by temperature. Kadlec and Knight (1996) suggest a θ value of 1.09 be used in equation 2.7 for denitrification in treatment wetlands.
Plant growth and nutrient uptake
Process: Many stormwater treatment practices include plants: algae in ponds; emergent aquatic plants in wetlands and ponds; and grasses and other plants in rain gardens, filter strips, and swales. Plants assimilate (take up) nutrients during growth. Algae growth can be represented (approximately) by equation 2.9. Algae are represented in this equation as a chemical formula: C106H236O110N16P.
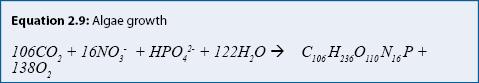
Equation 2.9 shows that photosynthesis (the forward reaction →) converts carbon dioxide (CO2), nitrate (NO3-), phosphate (HPO4-) and water to algae, producing oxygen (O2). Respiration (or death) is represented by the backward reaction (the reverse reaction ←). Respiration removes oxygen from the water while releasing carbon dioxide, nitrate, and phosphate to the water. For algae, periods of growth and senescence alternate in periods of a few weeks. Rooted aquatic plants obtain most of their nutrients from sediment during growth. When they decompose in late summer or fall, nutrients are released rapidly (days to weeks), which may result in increased nutrient concentrations in the water column (Landers 1982). A portion of nutrients remains in a refractory fraction, which becomes part of the sediment and decomposes slowly or not at all.
Assessment considerations: A large fraction of the nutrients assimilated by plants in stormwater treatment practices are released during decomposition. Most of the nutrient uptake by plants is therefore non-permanent. In wetland and pond systems, deposition of partially decayed (refractory) plant material will accumulate. Assessing the rate of accumulation can be important because plant debris will eventually need to be cleaned out. For example, Scheuler (1992) suggests that clean-out of wetlands is required at intervals of 2–10 years.
Coliform production and loss
Process: Fecal coliforms are excreted from the bodies of warm-blooded animals. For urban stormwater, sources may include humans (via illicit sewage connections to stormwater conveyances), dogs, cats, geese, and other wildlife. Although generation rates (number of coliforms excreted per day) for various organisms (dogs, geese, humans) are well known (Scheuler 2000b), there is little information regarding “delivery ratios” (the fraction of excreted coliforms that enters runoff) for urban stormwater.
Coliforms entering the natural environment die off according to equation 2.10. Values for k in streams are often on the magnitude of 1 d-1. Fecal coliforms can also regrow in the environment under warm conditions with a supply of organic matter for food, conditions that might occur in wetlands or stormwater ponds. Regrowth is not readily modeled in natural environments. Coliforms are readily removed by filtration during infiltration through soils, except in the case of very coarse soils.

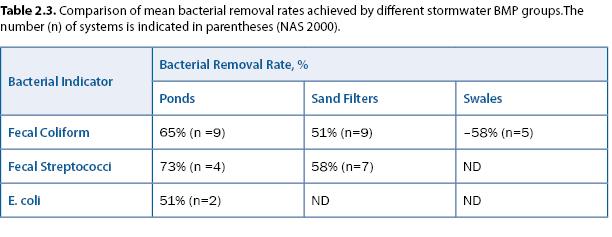
Assessment considerations: A study by the National Academy of Sciences (NAS 2000) noted that very large removal rates—on the order of 99%—would be needed to reduce coliforms from the levels observed in urban stormwater (15,000–20,000/100 mL) to EPA’s 200/100 mL criterion for recreational water. Their review indicated that bacterial removal rates in several types of stormwater treatment practices were significantly less than 99% (table 2.3). Studies of coliform regrowth in stormwater ponds have apparently not been reported in the peer-reviewed literature.
Continue to Chemical processes.